Ideal Gas Law in context of bottle
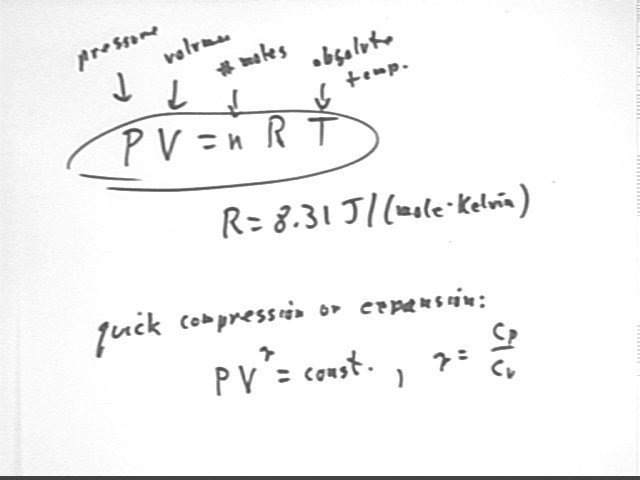
Ideal Gas Law
Squeezing a capped, empty soft drink bottle we easily experience the fact the decreasing volume is associated with increasing pressure.
According to the Ideal Gas Law, this corresponds to a decrease in V with n constant (because of the cap). If T is also constant we can conclude that P * V is constant and that P and V will be inversely proportional.
If we squeeze the bottle slowly, in a room where the temperature is the same as that of our hand, any tendency of the temperature to change will be negated by thermal energy transfers between the bottle and the room (i.e., the air in the bottle will always have time to absorb or give up enough heat to remain at constant temperature). In such a case we will have constant n and constant T and P will be inversely proportional to V.
If we squeeze the bottle quickly we are doing net work on the gas, which will increase the total KE of the gas particles, thereby increasing the temperature. In this case P, V and T will all be variable and the situation much more complicated than for constant T. The tendency of T to increase results in a more rapid pressure buildup than for constant T, resulting in the adiabatic gas law quoted, but not explained, below.
