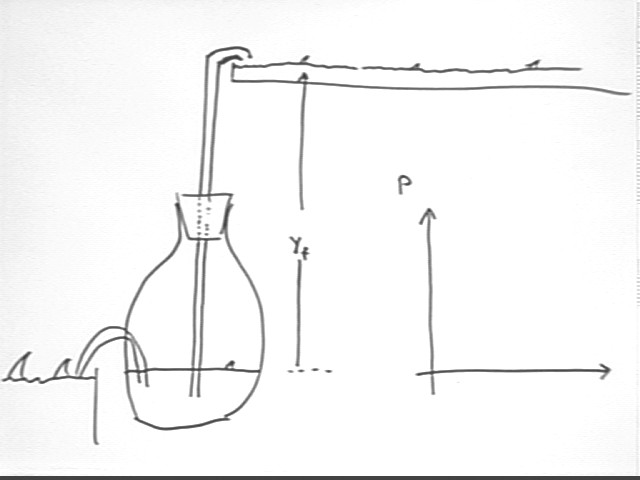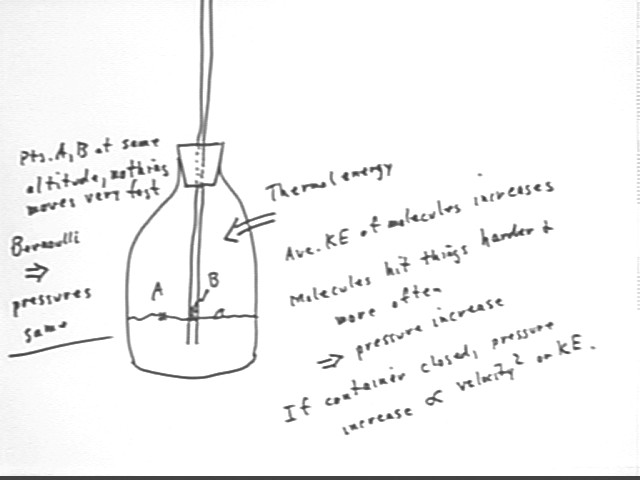
Raising water using thermal energy (bottle)
In the pictured container, points A and B are at the same altitude and we assume a slow process so that the velocities are insignificant. Thus `d(rho g h) and `d(1/2 rho v^2) are insignificant, and the pressure at A must equal the pressure at B.
If we increase the pressure at A, the only way pressure at B can equalize is for water to rise in the tube.

Suppose that we wish to lift water from one large reservoir to another which lies at altitude yf relative to the first. If the tube ends at altitude yf relative to the lower water level the the following observations hold:
The process of lifting the water increases the PE of the system. This required the addition of thermal energy to the system.
As the principle of energy conservation leads us to believe, we expect that the work done to lift the water will be less than the thermal energy put into the system.