"
Physics II
Class Notes, 1/27/99
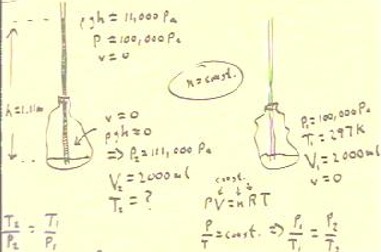
If the system depicted in the picture at left in the figure below
consists of 2000 ml of air, supporting a column of water
1.11 meters high, then assuming that the system started at
atmospheric pressure and 25 Celsius, what must be the temperature
of the gas inside the bottle?
- Using Bernoulli's's equation, a comparison of the state at the open end of
the tube and at the surface of the water in the bottle shows
us that the pressure in the bottle must be 11,000 Pa higher than
atmospheric pressure, or approximately 111,000 Pa.
- Since we assume that the tube is very narrow, with negligible
volume, we see that the volume of the system is
unchanging.
- Since the system is sealed from the outside, no gas can
enter or leave and we therefore see that the number of
moles n is constant.
- We therefore conclude from the ideal gas law PV = nRT that P / T = n R / V must
be constant, so that P1 / T1 = P2 / T2.
- Since we know both of the pressures P2 and P1 for the
two states of the system, and the temperature T1 of the original state of
the system, we can easily find T2.
To find T2 we invert the relationship P1 / T1
= P2 / T2 and plug in the appropriate quantities.
- We see that the temperature of the system must be 57 C.

If the system is now raised to a temperature of 80 C = 353 K,
with the water at the 1.11 meter level permitted to flow out of
the tube and into a reservoir that height, the gas will expand and
the water flowing out of the tube is that displaced by
the expansion.
- Since the height of the water in the open end of the
tube remains constant, the pressure of the gas remains
constant.
- Since air is blocked by water and therefore cannot bubble out through
the tube, it is clear that the number n of moles of air in the system
remains constant.
- We therefore conclude that only temperature T and volume V will
change.
- Intuitively we see that the ratio of volumes will be the same is the ratio
353 / 330 of temperatures during the expansion.
- We easily conclude that V2 / T2 = V1 / T1, where now state 1 is
the 'old' state 2, with pressure 1.11 kPa, volume 2000
ml and temperature 330 K and state 2 is the
state when temperature has reached 353 K.
- Since we know V1, T1 and T2, we can easily solve
for V2.
- We find that the volume of the system ends up at 2140 ml.
- The system will have therefore expanded by 140 ml, and
140 ml of water will have been displaced to
the higher reservoir.

We note that work has been done by this thermal engine, since 140
ml water has been raised to a height of 1.11 meters above
its original level.
- The work required to raise water is the product of its
1.3 Newton weight (140 ml of water has mass 140 grams and therefore
weight .14 kg * 9.8 m/s^2 = 1.3 N, approx.), and the 1.11 meter increase in
its altitude; the work is thus approximately 1.4
Joules.
- Looking at this from the standpoint of the energy of the system we see that the potential
energy of the system has increased by 1.4 J.
We wish to see how much thermal energy was transferred to the
system to accomplish this.
The thermal energy increase of the system is the
amount of thermal energy added to increase the temperature of
the gas.
- For a monatomic ideal gas, the amount of thermal energy required
to increase its temperature depends on whether the energy is added to a gas confined
to a constant volume, in which case no energy is required to expand
the gas, or at constant pressure, which implies an expansion
requiring additional energy.
- At constant volume, as indicated in the third line below,
the amount of thermal energy required to raise the temperature of n
moles of an ideal monatomic gas by temperature difference `dT is
3/2 n R `dT.
- At constant pressure the thermal energy required is greater,
since the gas must do work against the pressure in order
to expand. The amount thermal energy in this case is 5/2 n R `dT.
- We call the quantities Cv = 3/2 R and Cp = 5/2 R the molar
specific heats of an ideal monatomic gas at constant
volume and at constant pressure.

For a diatomic gas, such is the nitrogen or the oxygen
that makes up most of the air around us (and in our system), the
ideal values of Cv and Cp are 5/2 R and
7/2 R.
- The additional energy required for a diatomic gas is
the energy required to spin the dumbbell-shaped gas molecules end-over-end;
this energy is not needed for monatomic gasses.
From these molar specific heats we will be able to determine the
amount of thermal energy required to raise the temperature of
the gas in the system.
We will then be able to determine
the efficiency of our thermal engine, in terms of thermal energy
required and useful work done.
We can use our system to raise water to any height, up to the maximum
possible height dictated by the initial and final
temperatures 297 K and 353 K of the system.
- If we raise water to near the maximum height, we will have to 'use
up' most of our available temperature difference to increase
the pressure of the system at constant volume in order to get
the water to this height, and there will be very little temperature difference left
to expand the gas; as a result very little water will be
raised and not much useful work will
get done.
- If we raise the water only a little, very little
of the available temperature difference will be required to increase
the pressure at constant volume and get the water up the tube,
leaving a lot of temperature difference available to expand
the gas; as a result a lot of water will be raised
but since it is raised not very far not much useful work will
get done.
- Somewhere between the extremes of raising the water just a
little and raising it almost as much as possible, there is a height
to which we can raise the water to maximize the useful
work obtained from the system.
To determine the useful work done by the system, as temperature is
raised from 297 K to 353 K and water is raised to altitude
h, we consider the three states depicted below.
The first state is the initial state of the system,
the second the state when the water first reaches the required
altitude h, and the third the state when the final
temperature the system reaches 353 K.
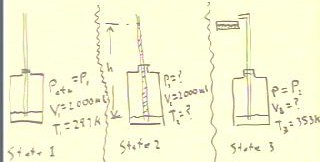
To determine the work done by the system we first find the volume
of the water raised:
- For a given altitude h, as we have seen in calculations done to this
point, we use Bernoulli's equation to determine the pressure P2 in
state 2.
- From the pressure P2 we determine the temperature T2,
using the known pressures P1 and T1.
- Then using the maximum temperature, T3, we use our knowledge of V2 and
T2 to find the final volume of the system.
- From the initial and final volumes we find the change
in the volume of the system.
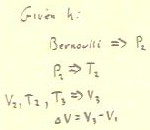
When applying Bernoulli's equation in state 2, we distinguish
between the state of the gas and the state of the water by
using A and B for the states of the water,
used in Bernoulli's equation. Otherwise we might become confused between state
1 and state 2 of the gas and the two
states of the water.
Looking at state 2, we let B be the state of the
water at the open end of the tube and A the state at the
surface of the water in the bottle.
Knowing every value of P, v and h except the pressure
at A inside the bottle, we substitute the known values and
easily determine this pressure in the bottle as
indicated below.
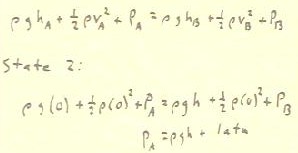
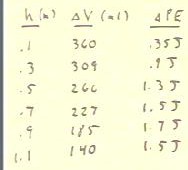
Following through the process we determine for various heights h the volume
changes `dV indicated below.
From each height and volume change we can then determine the associated
potential energy change.
- The results are shown in the table.
- We see that of the specified heights, the greatest potential
energy change occurs when we raise the water to a height of .9 meters.
It is not difficult to determine the amount of thermal energy required
to raise the n moles of gas from the initial to the final
temperature:
The system in state 1 consisted of 2000 ml = .002 m^3
of diatomic gas at 297 K and pressure 100,000 Pa.
- From PV = n R T we see that the number of moles is n = P V / (R
T), which for the conditions of state one tells us that there are approximately .08
moles of gas in the system.
- For every height there is a temperature at which the
system changes from constant volume to constant pressure.
- Knowing this transition temperature, the amount of thermal
energy transferred is found by using Cv as the molar specific
heat between original temperature and transition temperature,
then Cp between the transition temperature and the final
temperature of the system.
- To obtain a rough estimate of thermal energy required, we assume that
for the .9 meter height the transition temperature is
about halfway between the initial and final temperatures,
so the we can use an average of Cv and Cp
as our approximate molar specific heat.
- For diatomic gas this average is approximately 25 J / mol K.
Thus the energy required to heat the .08 moles from 297 K to
353 K, a change of 56 K, is about (25 J / (mol K) ) *
.08 moles * 56 K = 110 Joules (approx.).
- We see that we have obtained 1.7 J of useful work from
an input of 110 Joules of thermal energy.
- This implies an efficiency of 1.7 / 110, or about 1.6%.
Had we used lower initial temperature and/or higher final
temperature, assuming a container capable of withstanding these temperatures, we
would have been able to raise water to much greater heights using less
of the available temperature difference and, even though we
would have required greater thermal energy to increase the temperature
of the system, our gains in PE would have been even
greater and much greater efficiencies would have been possible.
"
