If we model the hydrogen atom as an orbital system with an electron orbit in the (much more massive) proton in a circular orbit, with the centripetal acceleration provided by the Coulomb force between the two unlike charges, we obtain the relationship between orbital velocity v and orbital radius r. If we assume that the angular momentum of the orbiting electron is quantized by the relationship m v r = n h / (2 `pi), where h is Planck's constant 6.64 * 10^-34 J s, we avoid the conclusion that the electron almost instantly dissipates all its energy in the form of electromagnetic radiation and we obtain a model that explains the spectra observed in discharging hydrogen. Solving the two resulting equations in v and r, we conclude that orbital radii of approximately rn = n^2 * .529 Angstroms are possible, with corresponding orbital velocities near vn = 1/n * (2 * 10^6 m/s). When an electron 'falls' from some allowed orbit to one with smaller radius its potential energy decreases and its kinetic energy increases, with a decrease in total energy. Energy is conserved by the emission of a photon with an associated frequency f = - `dE / h.
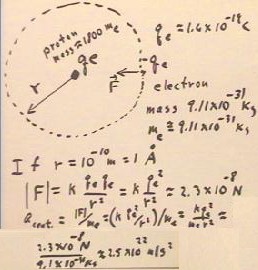
Just as a satellite can orbit a planet, with the centripetal acceleration supplied by the gravitational attraction between the planet and the satellite, we can think of an electron orbiting a proton, with the centripetal acceleration supplied by the electrostatic attraction between the charges.
- The proton has about 1800 times the mass of an electron (consult the tables in your book for the actual masses).
- For many applications we can assume that the center of mass of the system coincides with the proton, though for high-precision measurements and predictions the difference becomes significant.
The situation is depicted below.
- We note here that an accelerating electric charge will emit electromagnetic radiation, so that according to classical electromagnetism we would expect that the electron would emit a great deal of high-energy radiation, at the expense of its kinetic energy.
- That this does not happen will lead us to some of the basic assumptions of quantum mechanics.
In general we can set the electrostatic force F / me = k qe^2 / me r^2 equal to the centripetal acceleration v^2 / r.
We solve the resulting equation for v to find that
The proportionality constant `sqrt( k qe^2 / me) is easily evaluated. Its approximate value is 1.6 * 10^1 m^(3/2) / s.
This sort of motion would almost instantly dissipate the orbital KE of the electron in the form of electromagnetic radiation.
Since when a point mass m moves at velocity v in a circle of radius r its angular momentum I `omega is equal to m v r, we have the condition on angular momentum as indicated in the figure below.
Evaluating the quantity in brackets, we see that the radius of the orbit will be approximately n^2 * .529 Angstroms (an Angstrom is 10^-10 m).
The corresponding velocity is easily found to be approximately v = 1/n * (2 * 10^6 m/s).
For a given n, we thus have a specified orbital radius r and velocity v.
"