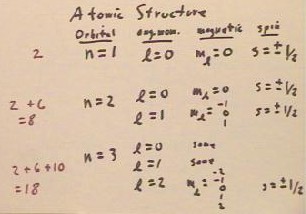"
Physics II, 4/28/99
Atomic Structure; Dual Nature of Light
The structure of an atom is largely governed by the values of the orbital, angular
momentum, magnetic and spin quantum numbers of its electrons. Every electron in an atom
must have a different combination of these four numbers.
By observing the relationships of the energies of electrons emitted when light
shines on certain metals as a function of the wavelength and intensity of the light, we
conclude that light delivers energy to the electrons in discrete amounts that depend on
the wavelength but not the intensity of the light. This behavior is consistent with the
behavior of particles but not of waves. We thus conclude that light sometimes acts as a
particle beam (with particles called photons having energies E = h f), and sometimes as
waves exhibiting diffraction effects and other wave properties. It has also been observed
that electron beams in certain circumstances exhibit wave behavior with wavelength `lambda
= h / (electron momentum) (this wavelength is called the deBroglie wavelength).
We can begin to understand atomic structure in terms of the quantization of four
quantities, the combination of which must be different for every electron in the atom.
- Each quantity is governed by a quantum number.
- The quantum numbers of an electron include:
- the orbital quantum number (the n that arises from the quantization of angular
momentum),
- an angular momentum quantum number having to do with the shape of the probability
distribution of the positions of a given electron,
- a magnetic quantum number which dictates the alignment of the electron orbit with an
external magnetic field, and
- a spin quantum number which dictates the alignment of the electron itself with an
external magnetic field.
The distribution of quantum numbers is governed by a few simple rules:
- The angular momentum quantum number may be any whole number less than the orbital
quantum number n.
- The magnetic quantum number may be any integer whose magnitude is less than or equal to
the angular momentum quantum number.
- The spin can be + 1/2 or - 1/2.
Using these rules we see that if n = 1, the angular momentum quantum number must be 0,
so that the magnetic quantum number (which has magnitude less than or equal to that of the
angular momentum quantum number) must be 0.
- There is therefore only one choice for these three quantum numbers.
- Since there are two choices for the spin quantum number, there are two possible
combinations for the n = 1 electrons.
The n = 2 electrons have the choice of angular momentum quantum numbers 0 or 1.
- If the angular momentum quantum number is 0, then the magnetic quantum number must be 0,
so that again there are only two choices for the quantum numbers.
- If the angular momentum quantum number is 1, then the magnetic quantum number can be -1,
0 or 1 and with the two possible choices for the spin quantum number we see that there are
6 possible combinations.
- Thus for n = 2 there are 2 + 6 = 8 possible combinations of quantum numbers.
A similar analysis works for the n = 3 orbital, with 2 and 6 possible combinations for
angular momentum quantum numbers 0 and 1, respectively.
- Angular momentum quantum number 2
is also possible, which gives rise to possible choices of -2, -1, 0, 1 or 2 for the
magnetic quantum number, and hence adds 10 possibilities for a total of 18 possible
combinations of quantum numbers.
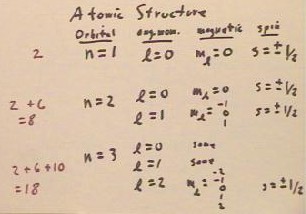
Video Clip #01
When single-wavelength light is shined on certain types of metal, called photoelectric
metals, there is a tendency for electrons to be emitted from the metal.
- The energies of these electrons can be measured by a setup such as that indicated in the
figure below.
- A wire mesh is maintained at a negative voltage with respect to the metal, tending to
repel the electrons that escape the metal.
- An electron therefore must have a certain kinetic energy to reach the grid.
- Upon reaching and passing through the grid, the electrons are collected on a metal plate
(not shown) and allowed to flow back to the photoelectric metal.
- By measuring the current of the electrons flowing back to the metal, we can measure the
rate at which electrons having sufficient energy to overcome the potential of the grid are
emitted from the metal.
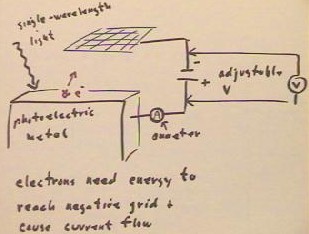
According to the wave theory of
light, brighter light should give electrons whose energy distribution peaks at a higher
energy.
- Thus more intense light should
eject higher-energy electrons from the metal, and for a given grid voltage the number of
electrons reaching the negative grid should increase continuously with the intensity of
the light.
- As the grid voltage is increase,
the observed current should therefore change in the manner depicted in the currect vs. V
graph.
- For a given voltage, the rate at
which electrons flow should thus be proportional to the area under the probability
distribution function for energies, starting at that given voltage (indicated for one
particular voltage in the graph at bottom right).
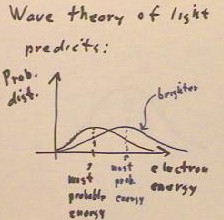
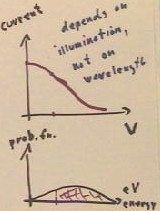
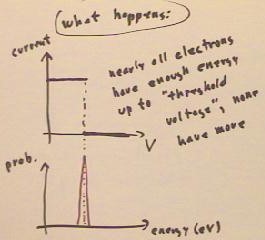
What is actually observed what is actually observed is that the current remains
constant up to a certain 'threshold voltage', indicating that practically all electrons
have an energy very nearly equal to that corresponding to this threshold voltage.
- The probability distribution
function is thus confined to energies very close to this threshold voltage, and we
conclude that the light imparts very nearly the same energy to every electron emitted.
- This energy does not depend on the
brightness of the light.
- Brighter light gives a higher
current, indicating that more electrons are emitted, but the threshold voltage does not
depend on the brightness.
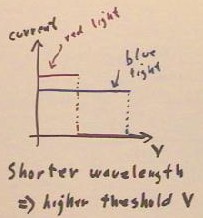
Video Clip #02
The threshold voltage does,
however, depend on the wavelength of a light. Blue light results in a higher threshold
voltage then red light.
- Shorter wavelength light implies a
higher threshold voltage, indicating that shorter wavelength light imparts greater energy
to the electrons.
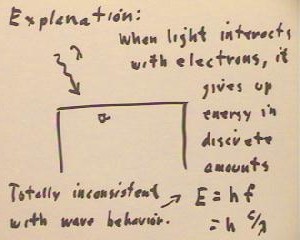
The conclusion drawn by physicists is that when the light interacts with the electrons
the gives up energy in discrete amounts, with the energy equal to the product of Planck's
constant h and the frequency f of the light wave.
- Since the frequency of the wave is inferred from the speed of light and the wavelength
(as measured by, say, diffraction gratings), we note that this energy could also be
written as E = h c / `lambda.
All this is totally inconsistent with any theory of wave behavior.
- It is as if the light is formed
from discrete particles, with the energy of each particle dependent only on the wavelength
of the light.
- But particle streams should not
exhibit wave behaviors such as refraction and interference effects.
- We are left with the uncomfortable
conclusion that light sometimes behaves as particles and sometimes as waves, and with a
sense that our intuitions about particles and waves is of little use in understanding
light.
If light with its wave behavior can sometimes act like a particle beam, then we might
expect that particle beams might be able to exhibit wave behavior.
This is in fact the case.
- An electron beam aimed at a
'diffraction grating' consisting of layers of atoms in a crystal exhibits interference
effects just as do light waves.
- The wavelength observed for a beam
of electrons is `lambda = h / ( electron momentum ), where the momentum of the electron is
the product of its mass and its velocity.

Video Clip #03
"