Physics II Class 01/10
indexed
What variables affect what happens when snow is mixed with water?
1. Relative amounts of snow and water
2. Temperature of snow, temperature of water, room temperature
3. Agitation (stirring)
4. Time of contact
5. Density, packing.
We have run an experiment which attempts to control for room
temperature, time of contact, density and packing. How did the design of the
experiment control for each of these variables?
Liquid water requires approximately 4.19 Joules per gram to increase by
1 degree Celsius. In order to decrease the temperature of 1 gram of water by 1
degree Celsius requires a loss of 4.19 Joules.
- How much thermal energy was lost by the water that was originally in the
cup?
- How much thermal energy was gained by the snow that was added to the cup?
- How much thermal energy was gained by the snow after it melted?
- How much thermal energy, per gram, was required to melt the snow?
Data taken by four different groups are given below:
| Initial water (grams) |
Initial water temp (Celsius) |
Snow added |
Final temp |
| 100 |
12 |
19 |
6 |
| 45 |
7 |
11 |
.2 |
| 94 |
7 |
21 |
10 |
| 80 |
14 |
18 |
5 |
The table below summarized the sequence of calculations:
- We first multiply 4.19 J per gram per Celsius by the temperature change
of the water in Celsius, to obtain the change in the thermal energy per gram of water.
For the first group we multiply 4.19 J / (gram Celsius) by the -6 Celsius
temperature change to get -25.14 Joules / gram.
- We then multiply this by the 100 grams to get -2514 Joules of energy
change.
- The energy lost by the water is presumably gained by the snow, since the
thermal energy change of the cup is negligible and the process was conducted too quickly
for significant thermal energy changes with the room.
- To get the thermal energy change of the melted snow we multiply its temp
change by the 4.19 J / (gram C). For the first group we obtain 4.19 J / (gram
C) * (+6 C); the result is then multiplied by the 19 gram mass to get 477.66 Joules (note
that the Excel calculation represented below does not round to the 2-significant-figure
accuracy of our observations; to avoid confusion we will use the Excel results here, but
you should note that the actual results should be appropriately rounded to represent the
appropriate number of significant figures.).
- The snow thus gains 2514 J of thermal energy, only 477.66 J of which goes
into raising the temperature of the melted water. We conclude that the difference,
2036.34 J, is required to melt the snow.
- Since this energy is required to melt 19 grams of snow, the energy per
gram is 2036.34 J / (19 gram). The result is represented as 107.1758 J / g in the
table; the appropriate result is rounded to 2 significant figures to give 110 J / g.
| mass
of water (g) |
init water temp (C) |
mass of snow (g) |
final temp (C) |
temp chg water |
water energy
change per gram |
total
energy change of water |
energy change of snow |
temp change after melt |
energy change after melt |
energy to melt snow |
energy to melt per gram |
| 100 |
12 |
19 |
6 |
-6 |
-25.14 |
-2514 |
2514 |
6 |
477.66 |
2036.34 |
107.1758 |
| 45 |
7 |
11 |
0.2 |
-6.8 |
-28.492 |
-1282.14 |
1282.14 |
0.2 |
9.218 |
1272.922 |
115.7202 |
| 70 |
34 |
21 |
10 |
-24 |
-100.56 |
-7039.2 |
7039.2 |
10 |
879.9 |
6159.3 |
293.3 |
| 80 |
14 |
18 |
5 |
-9 |
-37.71 |
-3016.8 |
3016.8 |
5 |
377.1 |
2639.7 |
146.65 |
We note that three of the four groups obtained results between 100 J / g
and 150 J / g. The third group obtained results at least double those of the other
three groups.
The accepted result is about 330 J / g. One likely explanation for
the discrepancies is thermal contamination of the snow, which was permitted by most groups
to sit in the room for several minutes before being mixed with the water.
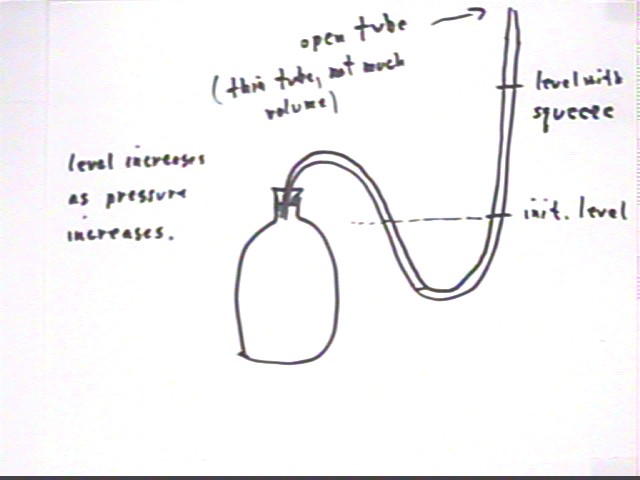
We also observed the following
For a column of alcohol in a thin U tube
(inner diameter 1/8 inch) partially filled with alcohol and extending from a sealed
3-liter bottle, represented by the figure below.
- When the top of the tube was open to the atmosphere a significant but
modest squeeze of the bottle raised the level of the alcohol about half a meter.
- When the top of the tube was closed a hard squeeze barely changed the
level of the alcohol.
- When the last half-meter of the tube was nearly level and open to the
atmosphere the temperature change induced by the instructor's palm in contact with, but
not squeezing, the container apparently caused the alcohol column to move a few
centimeters within a minute or so.