Physics II Quiz 01/27/03
Quiz
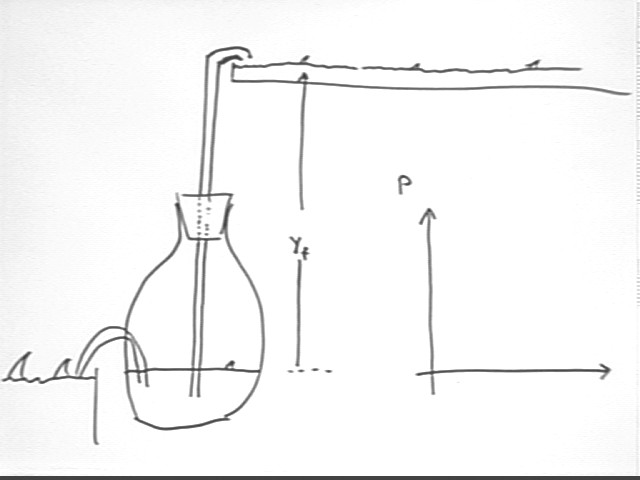
Consider the system indicated below.
The expansion of the gas is limited by the max temperature of the thermal energy source.
Once we get to max expansion we gotta cool off again if we want to pump more water.

We see that when thermal energy is put into this system in the specified manner the result is that mechanical work is done by the system and thermal energy is taken from the system in the cooling process.
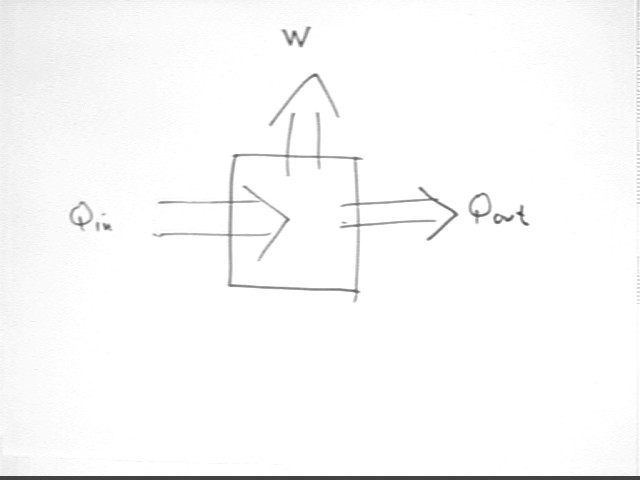
To better understand the system, and so that it will be possible to analyze the energy exchanges that take place, we must understand more precisely what happens to the energy that goes into the system and how the system is affected.
We begin by considering a closed container containing N 'billiard-ball' particles each of mass m, with all particles stationary. This corresponds to an initial state with no thermal energy; we say that the system is initially at absolute zero with respect to thermal energy.
We think of adding a quantity Q of thermal energy.

It follows that on the average the velocity of a particle is v = sqrt( 2 U / (m * N ) ).
We will see that knowing the average velocity of a particle and the volume of
the container we can find the average pressure exerted by the molecules. 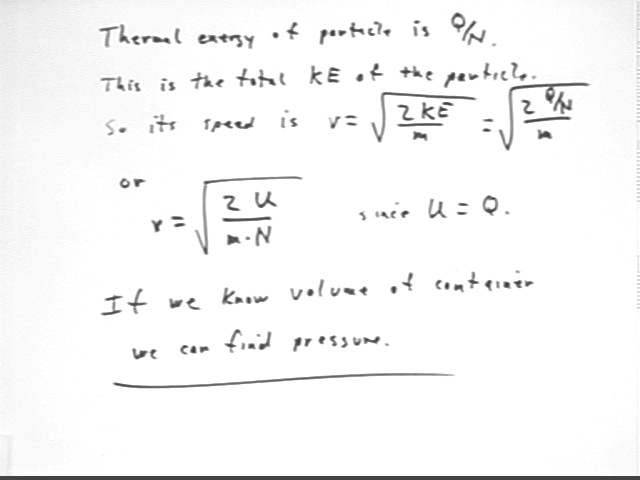
From the Introductory Problem Sets you are familiar with the fact that a single particle of mass m bounding back and forth between walls C and B of a container with velocity vx will exert force Fave = 2 * KEx / L on wall C.
If there are many molecules in the container they will collide with one another and on the average the x, y and z velocities will be equal, with vAve^2 = vxAve^2 + vyAve^2 + vzAve^2.

We therefore conclude that the force Fave exerted by a single particle is 2/3 KEave / L.
Since KEave = U / N we can rewrite this as
Rearranging these relationships we have

Since PV = n R T = N k T, with n, N, R and k as indicated below, it follows that
where KEave is the average KE of a particle.

For example, if 1 amu = 1.7 * 10^-27 kg, what is the ave velocity of this particle at T = 300 K?
We have KEave = 3/2 k T so
.5 mvAve^2 = 3/2 k T and
vAve = sqrt(3 k T / m) = sqrt((3 * 1.38 * 10^-23 J / (particle K) * 300 K ) / (1.7 * 10^-27 kg) ) = sqrt(7.2 * 10^6 m^2/s^2) = 2700 m/s.

The mass of an atom is the number of amu's equal to its atomic mass number. The atomic mass number of oxygen is 16. What would be the average velocity of a single oxygen molecule at 300 K?
Oxygen isn't normally monatomic. What is the average KE of a diatomic oxygen molecule?